Briefly Describe What Happens in the Photoelectric Effect
When an electron gets enough energy from a photon the electron can be ejected from the metal. Explaining the experiments on the photoelectric effect.

A Why Photoelectric Effect Cannot Be Explained On The Basis Of Wave Nature Of Light Give Reasons B Write The Basis Features Of Photon Picture Of Electron Magnetic Radiation On Which Einstein S Photoelectric
The effect is often defined as the ejection of electrons from a metal plate when light falls on it.

. In a broader definition the radiant energy may be infrared visible or ultraviolet light X-rays or gamma rays. It has three characteristics. 3 we see that the kinetic energy of the electrons is independent of the light intensity and depends only on.
Some materials exhibit a property known as the photoelectric effect that causes them to absorb photons of light and release electrons. Photoelectric effect describes the emission of electrons from the surface of a substance in response to incident light. Investigations of the effect of EM radiation on metals showed that electrons are emitted from the surface of a metal when EM radiation above a certain frequency was directed at the metal.
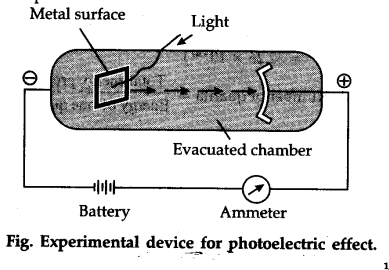
Explain briefly how the photoelectric effect is used in the operation of an electric eye. The photoelectric effect is the name given to the phenomenon of emission of electrons from a metal surface when the light of a suitable frequency is incident on it. Metals often show this property.
Briefly describe what happens in the photoelectric effect. The photoelectric effect is the process in which EM radiation ejects electrons from a material. Include the number of each ion you would need to draw so one formula unit of CaCl2 is correctly represented.
In fact light behaves both ways depending the situation. Predictions of the wave theory of light. How these experiments led to the idea of light behaving as a particle of energy called a photon.
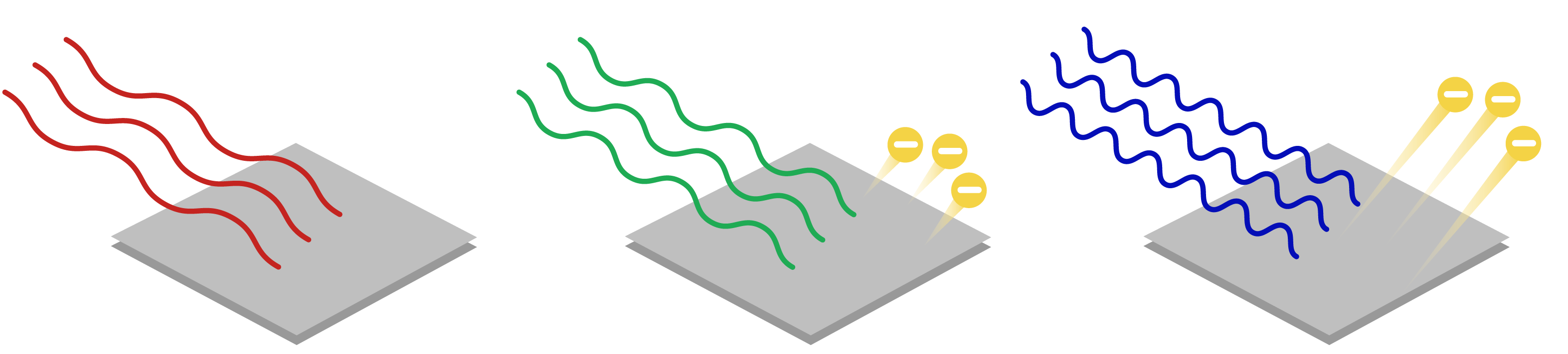
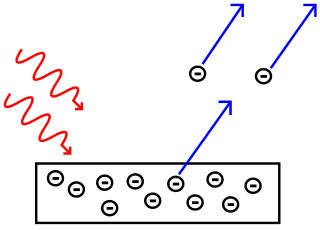
As soon as light hits the surface the electrons of the metal come out. The photoelectric effect is the process that involves the ejection or release of electrons from the surface of materials generally a metal when light falls on them. The Photoelectric Effect This topic is so important it deserves its own note set.
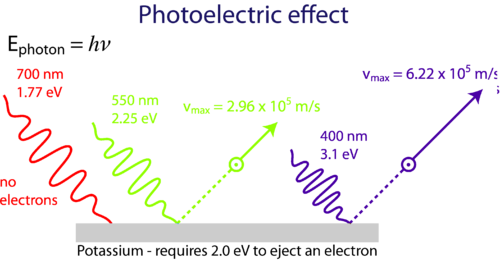
Einstein proposed photons to be quanta of EM radiation having energy E hf where f is the frequency of the radiation. Albert Einstein received the Nobel Prize for explaining the. 1 it is instantaneous 2 it occurs only when the radiation is above a cut-off frequency and 3 kinetic energies of photoelectrons at the surface do not depend of the intensity of radiation.
He explained that light comes in set amounts of energy and if it has enough energy the set of energy can remove an electron from an atom. The material may be a. The main difference between Photoelectric Effect and Photovoltaic Effect is.
The photoelectric effect is the most common form of interaction when the energy of the gamma rays is of the same order of magnitude as the energy binding atomic electrons to the nucleus. This is the currently selected item. Describe the orientation water around ions in CaCl2.
The gamma ray can then eject an electron away from an atom sharing its energy between the electron and the. However the electrons are bound in the metal so some of this energy must be used to overcome this binding. This is called the photoelectric effect meaning that light photo produces electricity.
There would be one Ca ion and two Cl ions. The Ca would have the negative O atoms attached and the Cl- will have the positive H atoms attached from H2O. The photoelectric effect is an important concept that enables us to clearly understand the.
For bound electrons their maximum kinetic energy will. The photoelectric effect occurs when light shines on a metal. One common use of the photoelectric effect is in light meters such as those that.
Photovoltaics is the direct conversion of light into electricity at the atomic level. 2 points A phenomenon in which electrons are ejected from the surface of a metal when light shines on the metal. When light strikes certain materials it can eject electrons from them.
Photoelectric effect phenomenon in which electrically charged particles are released from or within a material when it absorbs electromagnetic radiation. Plancks Theory and the Photoelectric Effect. When the incident light intensity is increased more photons are available for the release of electrons and the magnitude of the photoelectric current increases.
Photovoltaic effect is the process in which two dissimilar materials in close contact produce an electrical voltage when struck by light. Briefly describe the discovery of the photoelectric effect. When these free electrons are captured an electric current results that can be used as electricity.
The photoelectric effect is an instantaneous process. View Answer Light striking a metal plate can eject electrons from the plates surface called the. The more intense the light the more kinetic energy the.
How did Albert Einstein explain the photoelectric effect. The photoelectric effect occurs when photoelectrons are ejected from a metal surface in response to monochromatic radiation incident on the surface. Lets find out by studying the Experiment of the Photoelectric effect.
All EM radiation is composed of photons. This was the first instance of light interacting with matter so it was. The photoelectric effect shows that light behaves like a bunch of little particles but what about all the other important results like diffraction and interference that indicate light is a wave.
The Photoelectric Effect 8 points a. Light of any frequency will cause electrons to be emitted. In the photoelectric effect electrons are forced out from the surface of a metal when light shines on it.
Plancks theory was expanded by Einstein in 1905 to explain the photoelectric effect which is the release of electrons by metal when exposed to light or high photons. Sometimes electrons are emitted. A photon is absoberd an electron expelled from an atom.
In 1887 Heinrich Hertz discovered that certain metals emit electrons when light is incident on them. Google Classroom Facebook Twitter.
Photoelectric Effect Chemistry For Non Majors
Photoelectric Effect Article Photons Khan Academy

Photoelectric Effect An Overview Sciencedirect Topics

Photoelectric Effect Gizmo Solutions

Solved Q 5 Answer All Parts A Briefly Outline What Is Chegg Com

In The Photoelectric Effect Experiment A Beam Of Light Is Shining On A Metal Surface And The Electrons Are Emitted From The Metal One Of The Three Key Findings Is That A

Photoelectric Effect An Overview Sciencedirect Topics

Photoelectric Effect Definition Examples Applications Britannica
Photoelectric Effect Article Photons Khan Academy

Solved Briefly Describe The Photoelectric Effect And How The Chegg Com
What Is Photoelectric Effect Explain Briefly Cbse Class 11 Chemistry Learn Cbse Forum

What Is Photoelectric Effect State T He Result Of Photoelectric Effect Experiment That Could Not Be Explained On The Basis Of Laws Of Classical Physics Explain This Effect On The B Asis

In The Photoelectric Effect Experiment A Beam Of Chegg Com

Pdf A Research Based Curriculum For Teaching The Photoelectric Effect
Solved Q 5 Answer All Parts A Briefly Outline What Is Chegg Com

Solved Question 6 A What Is Photoelectric Effect Briefly Chegg Com



Comments
Post a Comment